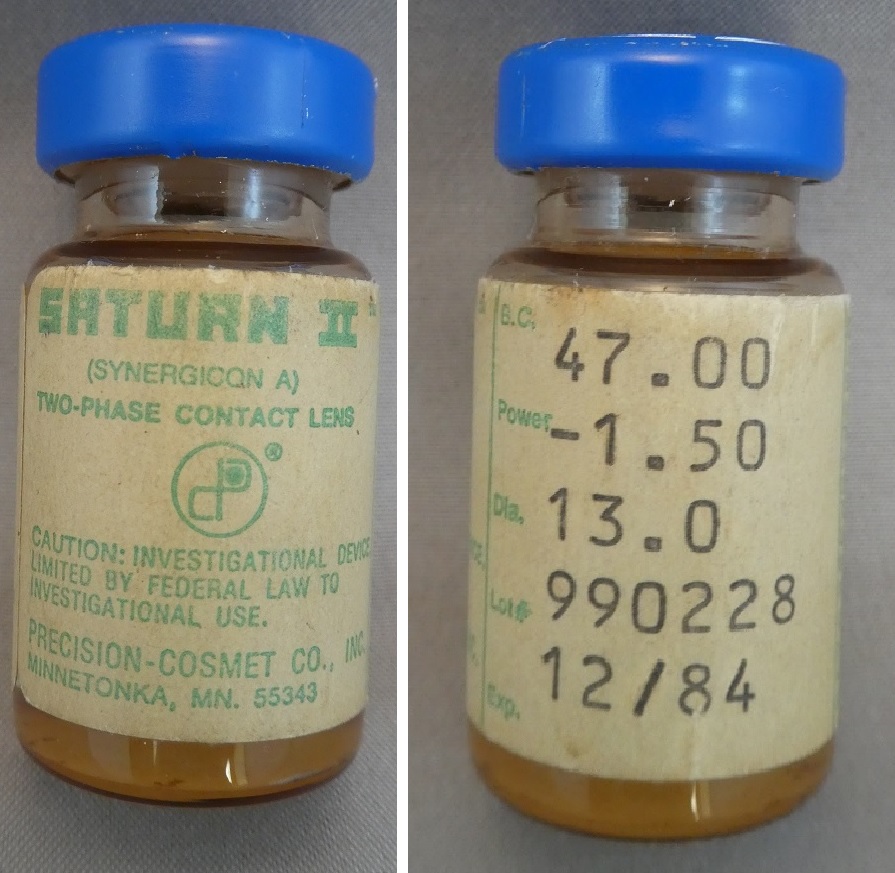
Saturn II Hybrid Contact Lens in Vial
Catalogue Number: 3921 Saturn II Hybrid Contact Lens in Vial Category: Spectacles and lenses Sub-Category: Contact lenses and accessories Corporation: Charles A. Erikson and Amar N. Neogi Year Of Publication/Manufacture: 1984 Time Period: 1940 to 1999 Place Of Publication/Manufacture: Minnetonka, Minnesota, USA Publisher/Manufacturer: Precision Cosmet Co. Inc. Description Of Item: Stoppered glass vial 48 mm x 22 mm diameter containing a Saturn II hybrid contact lens. Label affixed yellowed and imprinted in green Saturn II/ (Synergicon A)/Two-Phase contact Lens/Caution: Investigational Device Limited by Federal Law to Investigational Use/Precision-Cosmet Co Inc./Minnetonka, MN, 55343/ B.C. 47.00/Power -1.50/Dia. 13.0/Lot 990228/Exp 12/84/ Contents: One sterile Saturn II (synergicon A) Two Phase Contact Lens/ Hydrophilic Phase:25% water 75% synergicon A/ Lens immersed in buffered isotonic saline: CAUTION: Federal law prohibits dispensing without a prescription A1029A-82 Historical Significance: The Saturn II lens was the first truly hybrid contact lens. Patented by two scientists (Charles A. Erikson and Amar N. Neogi), it was acquired by Precision Cosmet Co., Inc. in 1977. It gained U.S. Food and Drug Administration (FDA) approval in 1984.It was not simply a GP lens 'attached' or glued to a soft lens skirt, but a molecular interweaving of the two different materials. Due to the low-Dk material and limited parameter options, the original lens often resulted in complications from tight fit and poor oxygen permeability.Sola Barnes Hind purchased the technology and developed the next-generation SoftPerm hybrid contact lens. It was more customisable and, therefore, more comfortable; however, issues of low Dk, lens tightness, adherence, and flexure persisted. Quarter Lambda Technologies Inc. (the predecessor to SynergEyes, Inc.) began to develop the next hybrid lens, which received FDA approval in 2005. The company's SynergEyes lens had many significantly improved properties. This has been further developed and the Duette line has addressed the low-Dk skirt concern in previous hybrid lenses. The lenses have a silicone hydrogel skirt with a Dk of 84 and a central GP lens Dk of 130. This lens is a possible choice for patients who have regular astigmatism and may require better stability, acuity, or more custom parameters than can be achieved in soft toric designs, or for those who may be unable to tolerate corneal GP lenses.From: Potter R OD FAAO Contact Lens Spectrum Vol 30 November 2015 pp 30, 32-35 How Acquired: Donated by Bruce Workman Date Acquired: 20-4-2019 Condition: Fair Location: Documents Room Small Red Cabinet Drawer 2 |